What Is Rheology?
Rheology is the study of the flow of materials and involves the flow properties of materials and whether they be liquid-like, solid-like, and intermediate compounds. The main behaviour that is of interest in rheology is the relationship between how material flows and force and the key characteristic which describes this which is viscosity. Another benefit of rheology is the ability to provide mathematical models for the behaviour of materials.
Theory of Rheology
Viscosity is the measure of a fluid’s resistance to flow and describes the internal friction of a moving fluid. The viscosity (m) (or dynamic viscosity which is more correct (equation 1). One of the best ways to visualize viscosity is by using a scenario such as fluid flowing between two plates (orange colour) with the bottom plate being stationary (figure 1).
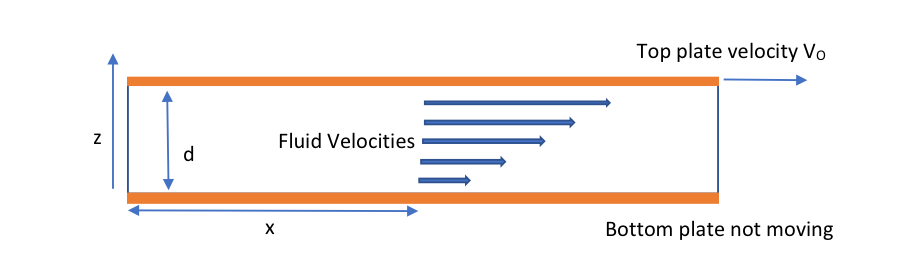
Figure 1: Fluid flowing between two plates
FA=μ(δu/δy)
Equation 1: Viscosity equation
μ – Viscosity
F – The shear stress or the force, F, required to move the plate divided by surface area in contact with the fluid
A- Velocity gradient or shear rate, where νo is the velocity of the plate and d is the thickness of the fluid layer
δu/δy – Rate of Shear Deformation
The units of viscosity are Pa.s which is the same as (N.s)/m2 or kg/(m.s)
What Is Shear Stress?
Shear stress is the force per unit area acting parallel to an infinitesimal surface element. The primary reason for shear stress is due to friction between fluid particles caused by different parts of a fluid relative to each other move at different velocities. When the shear stress is applied to a fluid at rest, the fluid will no longer remain at rest and will move because of the shear stress.
Consider a stream of flowing liquid in a pipe, the fluid velocity is zero at the pipe walls and will be the greatest towards the centre of the pipe, this shows that the adjacent layers of the fluid are moving at different velocities (figure 2). Within the fluid, there will be a continuous interchange of molecules between the flayers of fluid which will affect the velocities of the fluid layers, with molecules moving from a faster layer to a slower layer resulting in an acceleration in the adjacent fluid layers velocity and vice versa. This phenomenon is known as viscous drag and leads to viscous forces in adjacent layers within the fluid causing velocity changes. These forces are also known as viscous shear stresses and shear stresses in fluids are induced by fluid flow and the differing velocities within the flowing fluid. The effect of viscous shear stress is to reduce the velocity profile and between the fluid layers. Another way of thinking of shear stress is that it is a source of resistance to change in a fluid motion.
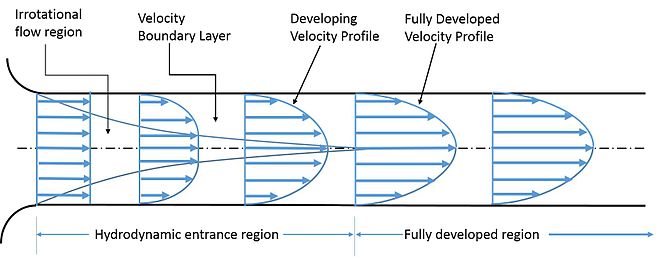
Viscosity Values
For liquids, viscosity is affected by temperature and not pressure, as the strength of the intermolecular forces changes with temperature and the greater the temperature the weaker the intermolecular force and shear becomes easier (table 1). Gases have low viscosities and are a function of both temperature and pressure.
| Fluid | Viscosity (mPa s) |
|
Water at 0°C | 1.79 |
|
Water at 20°C |
1.002 |
| Water at 100°C |
0.28 |
|
Glycerol at 0°C |
12070 |
|
Glycerol at 20°C | 1410 |
|
Glycerol at 30°C |
612 |
|
Gases |
0.01 |
| Sulphuric acid |
30 |
| Rubber compounds |
10,000,000 – 10,000,000,000 |
By seeing the values of the table, you can see how much resistance some fluids have towards flow such as rubber compounds, and how some fluids slow very easily, such as water.
Description Of Newtonian Fluids
Newtonian fluids obey Newton’s law of viscosity, where the shear rate and shear rate (the rate of change of the velocity of the fluid when one layer of fluid passes over an adjacent layer) is linear and the constant of proportionality is the viscosity. This means that the viscosity of the fluid remains constant regardless of changes to the rate of flow and any external stress from mixing or sudden application of force. Examples of Newtonian fluids are water, alcohol, air, glycerol, thin motor oils and some types of single-phase fluids made up of small molecules.
Description Of Non-Newtonian Fluids
Non-Newtonian fluids don’t obey Newton’s law of viscosity, and the viscosity which is referred to as apparent viscosity isn’t constant and it varies with the rate the fluid is sheared and the time the fluid is subjective to shear, this makes Non-Newtonian fluids a lot more complicated than Newtonian fluids.
Examples of shear rate dependant Non-Newtonian fluids are; pseudoplastic (shear thinning) where the viscosity decrease as the shear rate increases, dilatant (shear-thickening) the viscosity increases as the shear rate increases, Bingham plastic where there is no flow until critical shear stress is reached and then the rheogram becomes linear and Casson plastics that have no flow until shear stress is reached and then the rheogram is non-linear.
Examples of time dependant Non-Newtonian fluids are; Thixotropic (time dependant thinning) where the structure breaks down and viscosity decrease with increasing shear stress and Rheopectic (time dependant thickening) as the structure builds up the viscosity increases with the increase of shear stress.
A Rheogram Example
To visualize the flow characteristic of Newtonian and Non-Newtonian fluids a rheogram is used (figure 3). Programs are a plot of shear stress or sometimes viscosity against shear rate and are a collective of points collected from experimental data with a curve drawn through them. Sometimes calculations can be fitted to the curve to show the behaviours of fluids, but the calculations aren’t accurate outside of the conditions they are determined at.
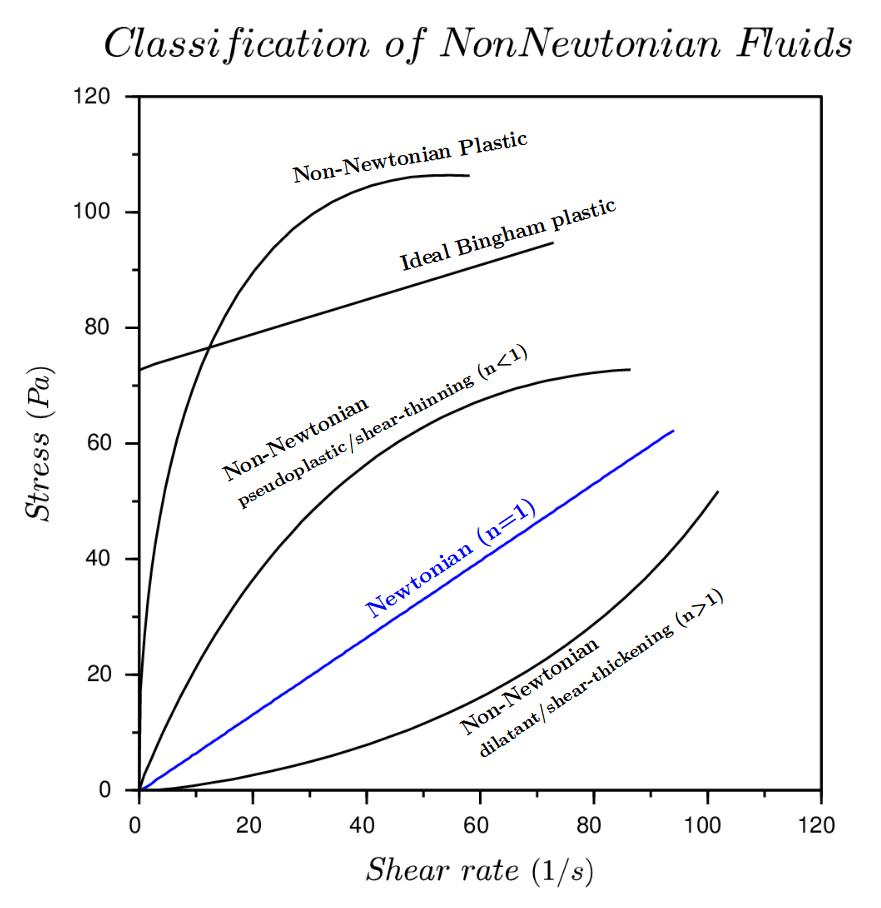
Remember: Viscosity is shear stress divided by the shear rate or the gradient of the line.
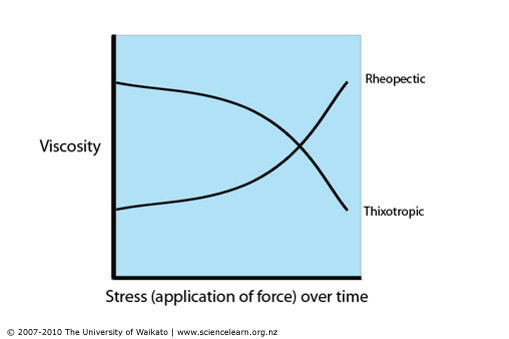
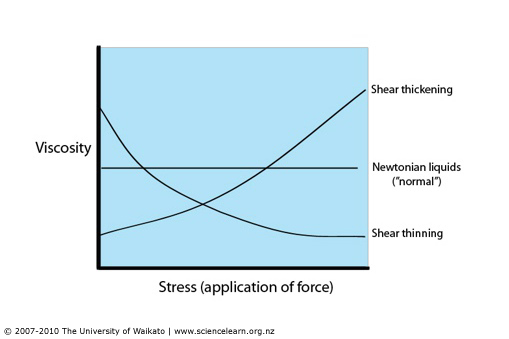
Figure 3: Rheogram showing Newtonian and Non-Newtonian fluids (Simscale, 2020) and rheogram using viscosity on the y-axis (Hub, 2020)
Examples of Newtonian and Non-Newtonian Fluids
| Fluid Type | Examples |
|
Newtonian | water, all gases, and low-molecular-weight liquids |
|
Non-Newtonian | |
| pseudoplastic |
Adhesives, rubber solutions, and polymer solutions |
|
Bingham plastics |
Margarine, cooking fats, and toothpaste |
|
Casson plastics |
Blood, tomato sauce (ketchup), and orange juice |
|
Dilatant |
A mixture of corn starch and water (a cooking agent used to thicken) and silica and polyethylene glycol (used in body armour) |
|
Thixotropic
|
Some types of clay, cytoplasm, and solder pastes (used in electronics manufacturing printing)
|
| Rheopectic
|
Gypsum, printer inks, and some types of lubricants.
|
References
Hub, S. L. (2020). Non-Newtonian fluids. Retrieved from Science Learning Hub: https://www.sciencelearn.org.nz/resources/1502-non-newtonian-fluids
Simscale. (2020). Non-Newtonian Models. Retrieved from Simscale: https://www.simscale.com/docs/simulation-setup/materials/non-newtonian-models/
Wikipedia. (2020). Entrance length. Retrieved from Wikipedia: https://en.wikipedia.org/wiki/Entrance_length

Dr. Adam Zaidi, PhD, is a researcher at The University of Manchester (UK). His doctoral research focuses on reducing carbon dioxide emissions in hydrogen production processes. Adam’s expertise includes process scale-up and material development.’



