Temperature is the sort of thing which you might think is simple and part of everyday life, however, the science behind temperature is far more complicated than that. This section delves into the physics behind temperature, exploring its ties to thermodynamics and statistical physics. Learn how temperature relates to the speed and momentum of atoms and molecules, and discover the deeper meanings behind what we perceive as hot or cold.
Temperature might appear to be a straightforward concept in daily life, but if we look a little deeper it starts to become a little more complicated and tricky. This article aims to explain the physics behind temperature and how it connects to thermodynamics and statistical mechanics. You’ll learn how temperature reflects the motion and energy of atoms and molecules, revealing the true nature of what we call “hot” and “cold.”
To briefly understand temperature, its important to understand the basics of thermodynamics. We recommend the following book for an introduction into thermodynamics:
What is Temperature?
In simple terms, temperature measures how fast atoms and molecules move within a substance. The higher the temperature, the quicker these particles vibrate or travel. This motion represents thermal energy, showing that heat is fundamentally a form of kinetic energy.
We can view this using the following idea. The faster the particle moves, the higher their energy and, therefore, the higher the temperature. What we measure as temperature is actually the average motion of microscopic particles.
Friction is a clear example of this concept. When two surfaces rub together, motion is converted into heat. The same happens in your body during exercise—your muscles generate heat as they work.
How Temperature Affects Pressure, Volume, and Other Physical Properties
Understanding temperature is crucial to understanding how matter behaves, and thus influencing pressure, volume, density, and even motion. A simple example is a balloon on a warm day. As temperature increases, the gas particles inside move faster, collide more often, and push outward; causing the balloon to expand.
This relationship between temperature, pressure, and volume is described by the ideal gas law, which shows how tightly these variables are linked. To learn more about Ideal Gases check out our article here. A rise in temperature reduces air density, explaining why warm air rises, why balls travel differently in hot weather, and why parachutes descend more slowly in warmer conditions.
Temperature also affects how materials conduct and store heat. Their specific heat and thermal conductivity determine how quickly they respond to temperature changes. You can view more on specific heat and thermal conductivity following the hyperlinks above.
Finally, temperature measurement itself varies across scales such as: Celsius, Fahrenheit, and Kelvin. Each one offers a different reference point. Understanding these scales and converting between them helps maintain global consistency in science, engineering, and daily life.
Temperature Scales Explained | Celsius, Fahrenheit, Kelvin, and How to Convert Between Them
When it comes to measuring temperature, not all “degrees” are created equal. While Celsius, Fahrenheit, and Kelvin are the most widely used scales, each measures temperature differently depending on its reference points. For example, water freezes at 0 °C, 32 °F, and 273.15 K but all describe the same physical state.
Outside of these familiar systems, there are several lesser known scales. Historical units like Rankine, Delisle, Newton, Réaumur, and Rømer were once used by the scientific communities one way or another before standardisation to our widely used units.
Our temperature conversion calculator includes all these units, letting you explore how each relates to the others.
The list of temperature conversions included in our calculator below are as follows:
- Celsius (°C)
- Fahrenheit (°F)
- Kelvin (K)
- Rankine (°R)
- Delisle (°D)
- Newton (°N)
- Réaumur (°Ré)
- Rømer (°Rø)
| Rankine | Kelvin | Fahrenheit | Celsius | Delisle | Newton | Réaumur | Rømer |
|---|---|---|---|---|---|---|---|
| °R | K | °F | °C | °D | °N | °Ré | °Rø |
| 0 | 0 | -459.67 | -273.15 | 559.73 | -90.14 | -218.52 | -135.9 |
| 100 | 55.56 | -359.67 | -217.59 | 476.39 | -71.8 | -174.07 | -106.73 |
| 180 | 100 | -279.67 | -173.15 | 409.73 | -57.14 | -138.52 | -83.4 |
| 459.67 | 255.37 | 0 | -17.78 | 176.67 | -5.87 | -14.22 | -1.83 |
| 491.67 | 273.15 | 32 | 0 | 150 | 0 | 0 | 7.5 |
| 559.67 | 310.93 | 100 | 37.78 | 93.33 | 12.47 | 30.22 | 27.33 |
| 671.67 | 373.15 | 212 | 100 | 0 | 33 | 80 | 60 |
The input value for the following conversion equations will be considered in Celsius, and then we can convert it to Fahrenheit, Kelvin, Rankine, Delisle, Newton, Réaumur, and Rømer.
Conversion From Celsius to Fahrenheit
Fahrenheit = Celsius x 9/5 + 32
Example: If Celsius = 100, then Fahrenheit = 100 x 9/5 + 32 = 212
Conversion From Celsius to Kelvin:
Kelvin = Celsius + 273.15
Example: If Celsius = 100, then Kelvin = 100 + 273.15 = 373.15
Conversion From Celsius to Rankine:
Rankine = (Celsius + 273.15) x 9/5
Example: If Celsius = 100, then Rankine = (100 + 273.15) x 9/5 = 671.67
Conversion From Celsius to Delisle:
Delisle = (100 – Celsius) x 3/2
Example: If Celsius = 100, then Delisle = (100 – 100) x 3/2 = 0
Conversion From Celsius to Newton:
Newton = Celsius x 33/100
Example: If Celsius = 100, then Newton = 100 x 33/100 = 33
Conversion From Celsius to Réaumur:
Reaumur = Celsius x 4/5
Example: If Celsius = 100, then Réaumur = 100 x 4/5 = 80
Conversion From Celsius to Rømer:
Romer = Celsius x 21/40 + 7.5
Example: If Celsius = 100, then Rømer = 100 x 21/40 + 7.5 = 60
Temperature Conversion Calculator | Convert Between Celsius, Fahrenheit, Kelvin, Rankine, Delisle, Newton, Réaumur & Rømer
The Celsius Scale Explained
The Celsius scale is one of the most widely used systems for measuring temperature. It is incredibly simple and practicale. Developed in the 1700s by Swedish astronomer Anders Celsius, it was based on two key reference points: the freezing and boiling temperatures of water.
Interestingly, Celsius originally set 0°C as the boiling point and 100°C as the freezing point. He later reversed to the version we use today. He divided the range between these points into 100 equal parts, creating what was once called the centigrade scale.
With its clear 0 to 100 structure, the Celsius scale became the standard for everyday temperature measurement in most countries (which use the metric system, SORRY USA!) and was long recognised as the official SI unit for practical use.
Advantages of The Celsius Temperature Scale
- International Standard: The Celsius scale is recognised worldwide and serves as the global standard for temperature measurement. It was the official SI unit until 2007, when Kelvin became the preferred scientific unit.
- Based on Water's Properties: Celsius is defined by the freezing and boiling points of water at 1 atm (0 °C and 100 °C), making it intuitive and practical for education and daily use.
- Ease of Conversion with Scientific Units: Celsius aligns directly with the Kelvin scale, where 0 °C equals 273.15 K. This simple relationship makes conversions effortless in scientific contexts.
- Simple and Practical for Daily Use: With round, easy-to-remember reference points for freezing and boiling, Celsius is ideal for everyday temperature readings.
- Compatibility with Metric System: As part of the metric system, Celsius integrates seamlessly with other metric units, supporting consistency in global science and engineering applications.
Disadvantages of The Celsius Temperature Scale
- Not Ideal for High-Precision Science: For extremely precise or high-temperature measurements, scientists prefer the Kelvin scale, which begins at absolute zero and eliminates negative values.
- Less Detail for Weather Reporting: Fahrenheit offers finer distinctions between temperatures near freezing and boiling, making it more descriptive for everyday weather reports in some regions.
- Limited Global Adoption: Celsius is not used everywhere. Countries like the United States still rely on Fahrenheit, requiring frequent conversions in global communication which can lead to mistakes - sometimes costly.
- Negative Temperatures in Cold Climates: Celsius often drops below zero in colder regions, which can feel less intuitive to the public than Fahrenheit, where typical weather values remain positive.
- Challenges with Historical Data: Older records in Fahrenheit must be converted to Celsius, which can introduce minor rounding errors in long-term climate datasets.
The Kelvin Temperature Scale Explained
The Kelvin scale was developed in 1848 by Lord William Thomson, later known as Lord Kelvin, a British physicist and engineer. He proposed an absolute temperature scale based on the fundamental laws of thermodynamics, where zero represents the complete absence of thermal energy.
Since then, the Kelvin scale has become the foundation of scientific temperature measurement. It increases in the same increments as Celsius but starts from a different zero point which is absolute zero (0 K), the theoretical temperature where all molecular motion stops. This point, equivalent to –273.15 °C, represents the lowest possible temperature in nature.
The Kelvin scale’s precision and independence from external conditions make it ideal for research. It’s defined using the triple point of water (See figure 1 below), where water exists as solid, liquid, and gas simultaneously, providing a stable reference for scientific calibration worldwide.
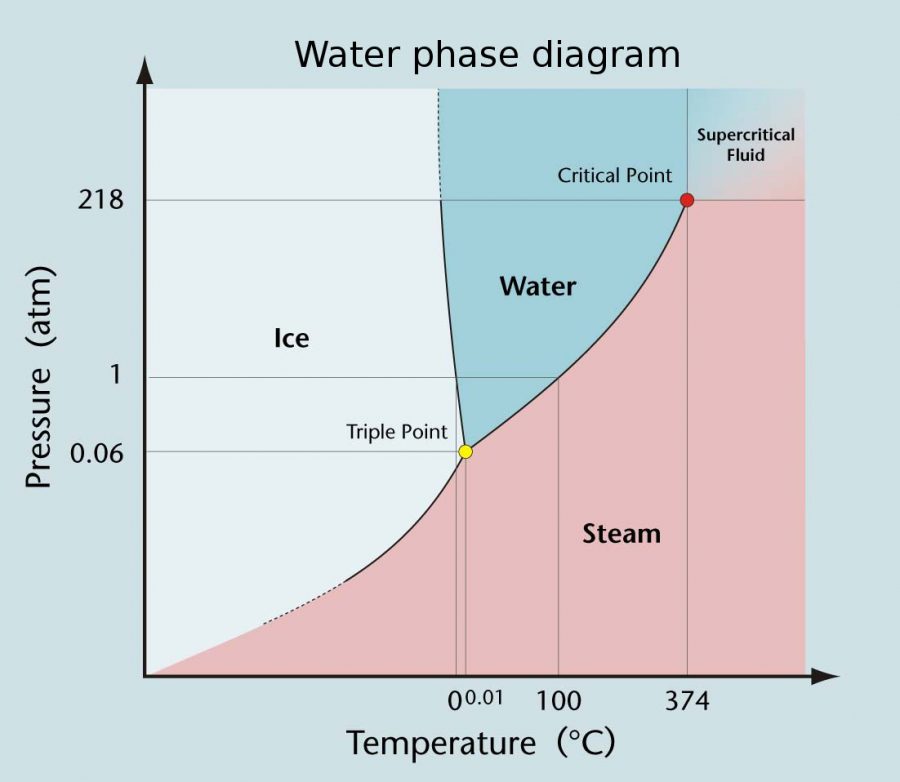
Advantages of Kelvin The Temperature Scale
- Absolute Zero as the Starting Point: The Kelvin scale begins at absolute zero. This is the point where all molecular motion stops, making it ideal for studying thermodynamics and understanding physical limits.
- Direct Link to Energy: A change of one Kelvin equals a change of one degree Celsius, allowing temperature shifts to be directly related to changes in energy in thermodynamic calculations.
- No Negative Values: Because it starts at absolute zero, the Kelvin scale has no negative numbers. This simplifies many scientific equations, especially in quantum and statistical physics.
- Scientifically Standardised: Kelvin is the official SI unit for temperature, ensuring global consistency and reliability in research and communication.
- High Precision: The Kelvin scale enables extremely accurate temperature measurements, crucial for experiments that demand exact thermal data.
Disadvantages of The Kelvin Temperature Scale
- Not Practical for Daily Use: The Kelvin scale isn’t suited for everyday use like weather reports or cooking, where Celsius or Fahrenheit are more intuitive for the average person.
- Large Numerical Values: Common temperatures appear as high numbers (e.g. room temperature ≈ 293 K), making Kelvin less convenient for routine use.
- Low Public Familiarity: Few people use or understand Kelvin, which can lead to confusion outside scientific settings and would be a hard barrier to overcome.
- No Everyday Reference Points: Unlike Celsius, which is based on the freezing and boiling points of water, Kelvin uses absolute zero, a concept not easily relatable to daily life for the average person.
- Requires Specialised Equipment: Measuring near absolute zero demands highly sensitive instruments, limiting its use to advanced scientific experiments.
The Fahrenheit Scale Explained
The Fahrenheit scale was developed by German born, dutch scientist Daniel Gabriel Fahrenheit in 1724, who wanted and sought a precise and consistent way to measure temperature. Influenced by Ole Rømer’s earlier work, Fahrenheit refined thermometer design and introduced his own scale, setting 32°F as the freezing point and 212°F as the boiling point of water.
Although less intuitive for those accustomed to Celsius, the Fahrenheit scale remains widely used, particularly in the United States, for its practical range in everyday weather and household applications. Its interesting to note that they no longer use Fahrenheit in both Germany or The Netherlands.
Converting between the two systems is simple:
- Celsius → Fahrenheit: (°C × 9/5) + 32
- Fahrenheit → Celsius: (°F − 32) × 5/9
These quick formulas make switching between the two scales straightforward.
Advantages of the Fahrenheit Temperature Scale
- More Precise for Everyday Use: The Fahrenheit scale offers finer temperature intervals than Celsius, making small changes in weather easier to notice in daily forecasts.
- Regional Familiarity: In the United States and a few other countries, Fahrenheit remains the standard, making it the most intuitive and widely understood system for local weather reports.
- Positive Range for Common Weather: Most everyday temperatures in Fahrenheit stay above zero, making it easier to interpret; anything below zero clearly signals extreme cold.
- Cultural and Historical Significance: Used for centuries in English-speaking regions.
Disadvantages of the Fahrenheit Temperature Scale
- Less Practical for Science: The Fahrenheit scale isn’t based on water’s freezing and boiling points, unlike Celsius. Because Celsius aligns directly with Kelvin which is the standard for scientific calculations, it’s preferred in research and engineering.
- Limited Global Use: Most countries use Celsius, making Fahrenheit less practical internationally. This often leads to confusion or the need for conversion in global communication and travel.
- Non-Intuitive Reference Points: Water freezes at 32°F and boils at 212°F, values that are less straightforward than Celsius’s 0°C and 100°C. This makes Fahrenheit harder to grasp in education and science.
- Cumbersome Conversion: Switching between Fahrenheit and Celsius requires a more complex formula, while Celsius and Kelvin conversions are simple additions or subtractions of 273.15.
The Rankine Temperature Scale Explained
The Rankine scale, introduced in 1859 by Scottish engineer William John Macquorn Rankine, was created to aid thermodynamic calculations during the Industrial Revolution. It uses Fahrenheit-sized degrees but begins at absolute zero, making it especially useful in engineering applications involving heat, energy, and gas laws.
Advantages of the Rankine Temperature Scale
- Consistency with Fahrenheit: The Rankine scale is compatible with the Fahrenheit scale, making it easier for industries and educational systems already using Fahrenheit to integrate temperature measurements in scientific calculations without needing to convert to Celsius or Kelvin.
- Absolute Scale: Like Kelvin, Rankine is an absolute temperature scale. This means 0 Rankine is absolute zero, making it useful in thermodynamic equations where absolute temperatures are required.
- Familiar Unit Size: The size of one degree Rankine is the same as one degree Fahrenheit. This familiarity is useful for those who are used to thinking in terms of fahrenheit.
- Useful in Certain Engineering Fields: Rankine is still used in some branches of engineering, particularly within the United States, where its compatibility with other Imperial measurement systems is advantageous.
Disadvantages of the Rankine Temperature Scale
- Lack of Global Use: The Rankine scale is not widely used outside the United States.
- Confusion with Other Scales: Since most of the world uses the Celsius and Kelvin scales, using Rankine can lead to confusion and errors in conversion.
- Redundancy: Given that Kelvin offers a similar absolute scale and is more widely accepted globally, Rankine can be seen as redundant.
- Less Intuitive for Everyday Use: For general public use, temperatures in Rankine can be less intuitive compared to Celsius or Fahrenheit.
The Delisle Temperature Scale
The Delisle scale, created in the early 1700s by French astronomer Joseph-Nicolas Delisle, measures temperature in reverse. It starts at the boiling point of water and decreases as it cools. Once used in Russia and parts of Europe, it offered an alternative approach to temperature measurement before Celsius became the standard.
Advantages of the Delisle Temperature Scale
- Historical Significance: The Delisle scale is of historical interest, providing insights into how temperature measurement has evolved over time. It is part of the rich history of science and thermometry.
- Unique Approach: Unlike most modern scales, the Delisle scale is inversely related to temperature – it decreases as the temperature goes up. This uniqueness can be intriguing for educational purposes, providing a different perspective on temperature measurement.
- Simplicity in Concept: The scale was originally based on fixed points of water boiling and freezing, which makes the concept behind it relatively simple and easy to understand. This can be helpful in teaching the basics of thermodynamics and scale calibration.
Disadvantages of the Delisle Temperature Scale
- Lack of Modern Relevance: The Delisle scale is obsolete in modern scientific and practical applications. It has been almost entirely replaced by more standard and universally accepted scales like Celsius, Fahrenheit, and Kelvin.
- Inverse Relationship: The scale’s inverse nature (where the numbers decrease with increasing temperature) can be counterintuitive, especially since it is contrary to the more commonly used scales today.
- Inconvenience in Conversion: Converting from Delisle to other temperature scales is not straightforward and requires a specific formula. This makes it less convenient compared to more directly relatable scales like Celsius and Fahrenheit.
- Limited Range and Precision: The scale may not be as precise as modern scales for a wide range of temperatures, especially in scientific research where high accuracy is required.
- Lack of Standardization: Since the Delisle scale is no longer in common use, any instruments based on this scale would lack standardization and calibration against modern temperature measurement devices.
In summary, while the Delisle temperature scale holds a place in the history of temperature measurement and provides an interesting alternative perspective, it is not practical for modern-day use due to its inverse nature, lack of precision, and the inconvenience posed in its application and conversion to other scales.
The Newton Temperature Scale
The Newton temperature scale, created by Isaac Newton around 1700, is one of the lesser-known temperature scales. Newton began his work by experimenting with a variety of substances that changed properties with temperature, such as linseed oil. He measured the rate at which these substances cooled down from a heated state to room temperature. Newton chose a reference point that was a mix of ice and water, which he defined as zero degrees. He then marked several other points, including human body temperature.
Advantages of the Newton Temperature Scale
- Historical Significance: The Newton scale holds historical importance as it was one of the earliest attempts to create a systematic approach to measuring temperature. Its development marked a significant step forward in the field of thermometry.
- Simple Conceptual Basis: Newton's approach to temperature was based on a simple, easy-to-understand concept. He used the rate of cooling of water and ice to define his scale, making it conceptually accessible even to those without an extensive scientific background.
- Foundational for Modern Thermodynamics: Newton's work laid the groundwork for future developments in thermodynamics and temperature measurement. His ideas influenced later scientists who developed more precise and practical temperature scales.
Disadvantages of the Newton Temperature Scale
- Lack of Standardization: The Newton scale was based on subjective reference points, such as the human body's temperature, which can vary. This lack of standardization led to inconsistencies in temperature measurement.
- Limited Range and Precision: Newton's scale was not as precise as modern scales like Celsius or Fahrenheit. Its limited range made it less practical for scientific research where precise and extensive measurements are necessary.
- Obsolete in Modern Use: The Newton scale has become obsolete and is rarely, if ever, used in modern science or industry. This obsolescence is due to the development of more accurate and universally accepted scales like Kelvin, Celsius, and Fahrenheit.
- Incompatibility with Modern Science: Modern scientific research requires high precision and uniform standards, which the Newton scale cannot provide. Its use today would be impractical, especially in fields that require precise temperature control and measurement.
- Lack of Recognition: The scale is not widely recognized or taught, leading to a lack of understanding and awareness about it. This obscurity further diminishes its practical utility in contemporary settings.
While the Newton temperature scale holds historical importance and contributed to the early understanding of temperature, its practical applications are limited in the modern world due to its lack of precision, standardization, and compatibility with current scientific needs.
The Réaumur Temperature Scale
The Réaumur temperature scale, created in the early 18th century by the French scientist René-Antoine Ferchault de Réaumur, is a historical scale that was mainly used in Europe, particularly in France, before the widespread adoption of the Celsius scale. Réaumur, fascinated by the natural world, sought to create a temperature scale based on the properties of water, a substance he considered fundamental.
He defined his scale in 1730, using the freezing point of water as 0 degrees Réaumur (°Re) and the boiling point as 80 degrees Réaumur. The choice of 80 was influenced by his desire to avoid fractions and simplify calculations. He used a spirit-filled thermometer for his measurements, believing it to be more accurate than mercury thermometers of that time.
Advantages of the Réaumur Temperature Scale
- Simplicity in Design: The Réaumur scale was designed with simplicity in mind. Its reliance on the properties of water made it intuitive and easy to understand during its time.
- Practicality in Certain Applications: In some specific industries, like brewing and cheese-making, the Réaumur scale found practical application due to its tailored design around biological processes.
- Historical Significance: It holds a place in the history of science, showcasing the evolution of temperature measurement and the diversification of scientific thought in the 18th century.
Disadvantages of the Réaumur Temperature Scale
- Lack of Universality: The Réaumur scale was not widely adopted outside of Europe, leading to issues of standardization and communication in scientific research.
- Limited Range and Precision: With a design centered around the boiling and freezing points of water, the Réaumur scale lacked the range and precision required for more sophisticated scientific applications.
- Obsolescence: The scale became obsolete with the advent of more accurate and universally accepted scales like Celsius and Fahrenheit. Its usage declined rapidly as these scales provided greater accuracy and consistency.
- Incompatibility with Modern Science: Modern scientific research demands a high level of precision and standardization, which the Réaumur scale cannot provide. This incompatibility led to its gradual disuse in the scientific community.
- Non-linear Relationship with Other Scales: The Réaumur scale does not have a linear relationship with the Kelvin scale, which is now the standard for scientific temperature measurement. This non-linearity complicates conversions and reduces its practicality in scientific contexts.
The Réaumur scale played a significant role in the early development of thermometry and had certain practical advantages in its time, its limited range, precision, and lack of universality however, have rendered it largely obsolete in modern scientific and practical contexts.
The Rømer Temperature Scale
The Rømer temperature scale, developed by the Danish astronomer Ole Rømer in 1701, is one of the earliest attempts to quantify temperature. Rømer, known mainly for his astronomical work, particularly his calculations of the speed of light, ventured into thermometry influenced by his scientific environment and the burgeoning interest in temperature measurement during that era.
Rømer's motivation was practical. Before his invention, there wasn't a standardized way of measuring temperature. He wanted a consistent method to compare temperatures, crucial for scientific experimentation and daily life. His scale was a pioneering effort to bring a systematic approach to thermometry.
Rømer’s scale was based on two fixed points. He set the freezing point of water at 7.5 degrees and the boiling point at 60 degrees. The most distinctive feature of this scale was the third fixed point, which he established at 22.5 degrees, based on the temperature of brine (a saltwater solution). This point was the primary reference in his scale, rather than the freezing or boiling point of water, as seen in later scales.
Advantages of the Rømer Scale
- Innovative Approach: Rømer's introduction of a third fixed point was revolutionary. It provided a more diversified reference framework for temperature measurement during its time.
- Foundation for Future Scales: It laid the groundwork for future temperature scales. Rømer’s ideas influenced later scientists like Fahrenheit, who refined and expanded upon these concepts.
Disadvantages of the Rømer Scale
- Lack of Precision: By modern standards, the Rømer scale lacks precision. The arbitrary choice of brine as a reference point introduces variability, as brine's freezing point can change based on its salt concentration.
- Limited Scale Range: The scale had a limited range and was not suitable for measuring extremely high or low temperatures, limiting its scientific application.
- Obsolescence: Over time, more accurate and universally applicable scales like Celsius and Fahrenheit replaced the Rømer scale, leading to its obsolescence.
The Rømer scale is not used today, its historical significance lies in its role as a stepping stone in the evolution of thermometry. It represents an early and important attempt to bring a systematic approach to the measurement of temperature, paving the way for the more precise and practical scales that we use today.
Temperature Measurement: The How-To of Detecting Heat
Ever wondered how we actually figure out the temperature of something? It's a common question, and the answer lies not in measuring temperature directly – that's a bit tricky – but rather in observing its effects on other things, like how much a material expands, the pressure changes, or even the resistance in an electrical wire.
Let's break it down. One traditional method is using thermal expansion. This is pretty straightforward: most things get a bit bigger when they heat up. Picture a classic mercury or alcohol thermometer – it's all about how much the liquid inside expands. But this method isn't perfect. It's got a limited temperature range, needs direct contact with whatever you're measuring, and let's be real, it's not going to hook up to your smartphone for a digital readout.

Enter the modern era of temperature sensing. We've got gadgets that rely on how temperature affects a material's electrical properties. Think about electronic sensors that measure changes in voltage or resistance when things heat up or cool down. These include fancy stuff like thermocouples, which use the Seebeck effect – a phenomenon where different metals joined together generate a voltage when heated.
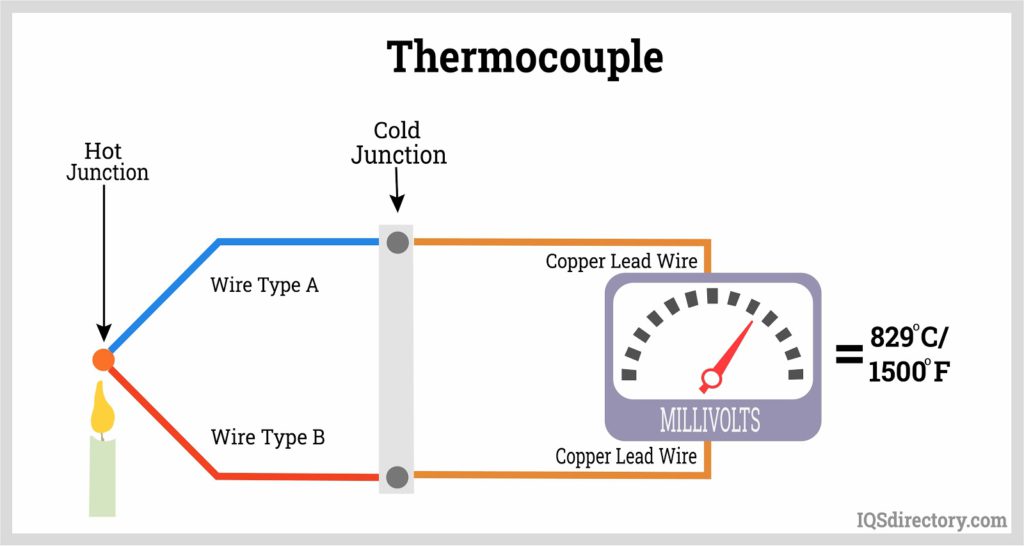
But wait, there's more! Ever wondered how scientists figure out the temperature of stars or even black holes? They use the color of the light these celestial bodies emit, which changes with temperature. This technique, based on the Stefan-Boltzmann and Wien's laws, translates the color spectrum into a temperature reading – pretty cool, right? This is similar to those infrared thermometers, shaped like guns, which measure the heat emitted by objects from a distance.

Sure, there are other ways to measure temperature, using more complex scientific principles, but these are often overkill for everyday needs. Most of the time, we stick to the methods that are practical and efficient for our daily use.
In essence, temperature is a crucial aspect we measure regularly, and we've become quite good at it. Although there's still some debate over which temperature unit reigns supreme, we've definitely nailed down the art of measuring those hot and cold vibes!

Hassan graduated with a Master’s degree in Chemical Engineering from the University of Chester (UK). He currently works as a design engineering consultant for one of the largest engineering firms in the world along with being an associate member of the Institute of Chemical Engineers (IChemE).



