The History of the Ideal Gas Law
The ideal gas law, given by ( PV = nRT ), is based on several key assumptions about the behavior of gases. These assumptions simplify the complex nature of real gases and make the law applicable under ideal conditions. The assumptions are as follows:
- Large Number of Molecules:
- The gas consists of a large number of molecules that are in constant, random motion. This assumption ensures statistical averages can be applied.
- Point Particles:
- The molecules of the gas are considered point particles, meaning they have negligible volume compared to the volume of the container. Essentially, the size of the gas molecules is much smaller than the distance between them.
- No Intermolecular Forces:
- There are no attractive or repulsive forces between the molecules except during elastic collisions. This means the potential energy of interaction between molecules is zero.
- Elastic Collisions:
- All collisions between gas molecules, and between molecules and the walls of the container, are perfectly elastic. This implies that there is no loss of kinetic energy in the collisions: Total kinetic energy before collision = Total kinetic energy after collision
- Newton’s Laws of Motion:
- The molecules obey Newton’s laws of motion, which means their behavior can be described using classical mechanics. This includes the conservation of momentum and energy.
- Random Motion:
- The molecules are in random motion and the distribution of their velocities follows the Maxwell-Boltzmann distribution.
These assumptions collectively define an “ideal” gas, which is a useful model for understanding the behaviour of real gases under many conditions, particularly at low pressures and high temperatures where real gases tend to show behaviours closer to ideal gases.
The development of this law is rooted in the contributions of several scientists over centuries:
- Boyle’s Law (1662):
- Robert Boyle, an Irish physicist and chemist, discovered that the pressure (P) of a gas is inversely proportional to its volume (V) at constant temperature. This relationship is known as Boyle’s Law:
PV = constant
- Charles’s Law (1787):
- Jacques Charles, a French scientist, found that the volume (V) of a gas is directly proportional to its temperature (T) when pressure is held constant. This relationship is termed Charles’s Law:
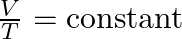
- Gay-Lussac’s Law (1809):
- Joseph Louis Gay-Lussac, a French chemist and physicist, established that the pressure (P) of a gas is directly proportional to its temperature (T) at a constant volume. This is known as Gay-Lussac’s Law:
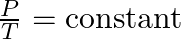
- Avogadro’s Hypothesis (1811):
- Amedeo Avogadro, an Italian scientist, proposed that equal volumes of all gases, at the same temperature and pressure, contain an equal number of molecules. This led to the concept of the mole and Avogadro’s number.
- Development of the Combined Gas Law:
- The relationships described by Boyle, Charles, and Gay-Lussac were integrated into the combined gas law, which relates pressure, volume, and temperature without changing the amount of gas:
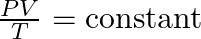
- Ideal Gas Law (1834):
- Émile Clapeyron, a French engineer and physicist, combined Boyle’s, Charles’s, and Avogadro’s laws into a single equation of state for an ideal gas. He introduced the ideal gas law in the form:
PV = nRT - Here, (R) is the ideal gas constant, (n) is the number of moles of gas, (P) is the pressure, (V) is the volume, and (T) is the temperature in Kelvins.
The ideal gas law encapsulates the behaviour of gases under a variety of conditions and forms the basis for more advanced gas theories and real gas behaviour corrections.
What Is An Ideal Gas?
An ideal gas is a theoretical gas that perfectly follows these conditions:
- The gas comprises a large number of molecules that move randomly.
- All molecules are point particles (they occupy no space).
- The molecules do not interact except during collisions.
- All collisions between gas particles are perfectly elastic (see our conservation of momentum calculator for more).
- The particles obey Newton’s laws of motion.
Ideal gas law equation
The properties of an ideal gas are summarised by the equation:
![]()
where:
- (p) — Pressure of the gas, measured in Pa;
- (V) — Volume of the gas, measured in m³;
- (n) — Amount of substance, measured in moles;
- (R) — Ideal gas constant;
- (T) — Temperature of the gas, measured in kelvins.
Ideal gas constant
The gas constant (R) is also known as the molar or universal constant. It appears in many fundamental equations, such as the ideal gas law. The value of this constant is:
R = 8.31446261815324 J/(mol·K)
The gas constant is often defined as the product of Boltzmann’s constant (k), Which can be worked out using our online calculator here. and Avogadro’s number (NA):
Ideal Gas Law Calculator
To use the calculator, select the variable you wish to find from the “Select Variable to Calculate” dropdown menu. Once selected, leave the corresponding cell blank e.g. if pressure is selected leave the pressure field blank. Then proceed to enter the other 3 variable and the answer will reveal itself in the calculated value cell.
When Can I Use The Ideal Gas Law?
The ideal gas law applies to gases at low densities, where intermolecular forces are negligible. Under these conditions, any gas can be approximately modelled by the equation ( PV = nRT ), which relates pressure, temperature, and volume.
Industrial Applications of The Ideal Gas Law
The ideal gas law, is essential in various industries for predicting gas behavior under different conditions. Key applications include:
Chemical Industry
- Reactions and Processes: Calculating gas volumes and pressures in chemical production and reactor design.
Petrochemical and Oil Industry
- Gas Recovery and Processing: Designing compressors and pipelines for natural gas and hydrocarbons.
Pharmaceuticals
- Synthesis and Quality Control: Managing gas conditions during drug synthesis and ensuring quality control.
Food and Beverage Industry
- Packaging and Preservation: Determining the pressure and volume of gases for packaging and carbonation processes.
Environmental Engineering
- Air Pollution Control: Designing emission control systems and predicting pollutant behavior.
Aerospace and Aviation
- Aircraft and Spacecraft Design: Managing gas mixtures and pressures in cabins and analyzing jet engine performance.
HVAC (Heating, Ventilation, and Air Conditioning)
- System Design and Optimization: Ensuring proper airflow and optimizing energy efficiency.
Metallurgy and Material Science
- Gas Metal Reactions: Controlling gas-metal reactions in processes like carburizing.
Manufacturing and Mechanical Engineering
- Pneumatics and Control Systems: Designing pneumatic systems for automation and manufacturing.
These applications demonstrate the ideal gas law’s versatility in efficiently designing, optimising, and controlling gas-involved systems across various industries.
What Is The Formula of The Ideal Gas Law?
The ideal gas law formula is:
![]()
where:
- (p) — Pressure of the gas, measured in Pa;
- (V) — Volume of the gas, measured in m³;
- (n) — Amount of substance, measured in moles;
- (R) — Ideal gas constant;
- (T) — Temperature of the gas, measured in kelvins.
Ensure to use consistent units! The common value for R (8.314 J/(mol·K)), refers to pressure measured in pascals.
What Are The Three Thermodynamic Laws Related To The Ideal Gas Law?
The ideal gas law involves four parameters, but three are directly related to thermodynamics: pressure, temperature, and volume. By determining each one, we identify three laws:
- Determining temperature finds the isothermal transformation. This is known also as Boyle’s law: PV = k.
- Determining volume finds the isochoric transformation. This is known also as Charles’s law:
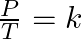 .
. - Determining pressure finds the isobaric transformation. This is known also as Gay-Lussac’s law:
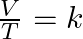 .
.
How Do I Calculate The Temperature Of A Gas Given Volume, Moles, And Pressure?
To calculate the temperature:
- Calculate the results of pressure multiplied by the volume using the units pascals and cubic meters.
- Calculate the results of the number of moles multiplied by the gas constant. When using pascals and cubic meters, the constant is R = 8.3145 J/(mol·K).
- Divide the result of step 1 by the result of step 2 to find the temperature in kelvin:
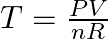 .
.

Hassan graduated with a Master’s degree in Chemical Engineering from the University of Chester (UK). He currently works as a design engineering consultant for one of the largest engineering firms in the world along with being an associate member of the Institute of Chemical Engineers (IChemE).



