What Is Ammonium Nitrate?
Ammonium nitrate is typically a white solid and is the salt produced from the reaction of ammonia and nitric acid (Figure 1). The ammonia is the product of the reaction between nitrogen and hydrogen, the two main hydrogen production process for ammonia are natural gas and coal which is predominately used in China.
The ammonia feed for the production of ammonium nitrate is liquid anhydrous ammonia, it is stored as a liquid under pressure to prevent it from escaping to the atmosphere as it becomes a toxic gas. This is the main issue with using anhydrous ammonia and so it must be stored in a safe and secure location (Dana A. Shea, 2013). The nitric acid that is used in the production of ammonium nitrate can be feed into the process; similar to ammonia, or it can be made during the process by reacting nitrogen dioxide and water. There are issues with nitric acid due to it being corrosive and a strong oxidising agent, therefore it must be stored securely so it is not exposed to other substances, so it is not able to react and explode.

What Are Some of The Properties Of Ammonium Nitrate?
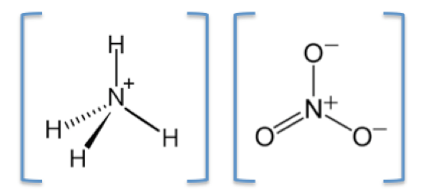
Ammonium nitrate is soluble in water, it is highly hydroscopic and is not very reactive, and is stable on its own. The chemical symbol of Ammonium nitrate is NH4NO3 its molar mass is 80.043 g/mol, it has ionic bonding and contains two ions: a cation, the ammonium ion (NH4+) and an anion, the nitrate ion (NO3–) (Figure 2). The boiling point of ammonium nitrate is 210 deg and its melting point is 169.6 deg and it has a density of 1.72 g/cm3 (Pubchem, 2018). Ammonium nitrate was discovered in 1659 by a German chemist called Johann Rudolf Glauber.
Ammonium nitrate doesn’t regularly occur in nature due to it being soluble in water and is easily washed away by rainwater, it has been found in certain desert regions however it is found as a mixture with other minerals, but this is rare (Encyclopedia, 2018). Ammonium nitrate is mostly sold as a solid, the two processes to produce solid ammonium nitrate are prilling and granulation. Prills are produced by sending concentrated ammonium nitrate, which is known as melt, down a prilling tower leaving the bottom of the tower as a solid. Granulation involves using a rotary drum to spray small seed particles of ammonium nitrate with melt to produce granules (United States Environmental Protection Agency, 2018).
The Ammonium Nitrate Market
The ammonium nitrate global market size was estimated to be $4.67 billion in 2016, and the global production is estimated to achieve 1700 kilotons by 2022 (Global Information, 2018). The reason that the ammonium nitrate market is so large is due to the high demand for fertilisers and explosives around the world. The ammonium nitrate market is dominated by Europe, the USA, and China in terms of production and consumption, with over 70% of consumption and over 80% of production occurring in these regions (Grand View Research, 2017).
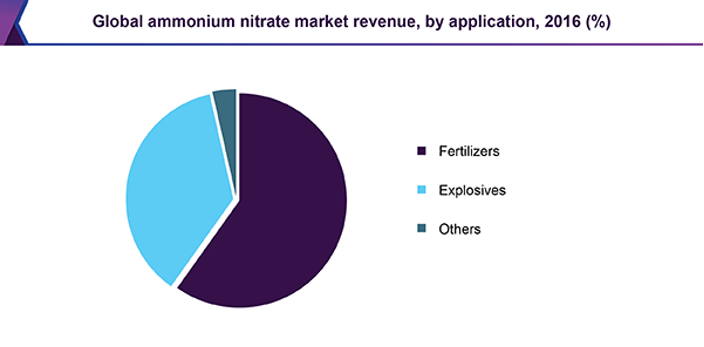
In today’s market ammonium nitrate prill has two primary applications, the main use of ammonium nitrate prill is in fertilisers, which accounted for nearly 60% of the ammonium nitrate market in 2016 (figure 3). The other main use of ammonium nitrate is in explosives. Ammonium nitrate prill is very important for the agricultural industry as it is one of the key components in fertilisers.
Fertilisers are typically made up of nitrogen, phosphorous, potassium compounds, and other trace elements (madehow, 2018). Fertilisers work by replacing the chemical components that are taken up by the plants from the soil, this leads to an optimum growing environment for the plants. Ammonium nitrate prills are highly soluble in soil and contain a high nitrogen content (about 33.5%). Nitrogen is an important nutrient for plants as it is a major component of chlorophyll, the compound by which plants use sunlight energy to produce sugars from water and carbon dioxide.
Furthermore, nitrogen is a major component of amino acids that make up proteins, and if a plant doesn’t have proteins it will die. Furthermore, nitrogen is a significant component in nucleic acids such as DNA that allows cells to grow and reproduce (Crop Nutrition, 2018). Therefore, it is evident that nitrogen is very important for plants and with Ammonium nitrate prill having such a high nitrogen content and being inexpensive it is very useful for the agricultural industry.
Ammonium nitrate prill is used in the manufacturing of explosives, this is due to it being an oxidizing agent and its explosive nature when it reacts with other compounds. ANFO is an extremely explosive compound and it is formed from the mixture of 94% prilled ammonium nitrate (AN) and 6% Fuel Oil (FO) (Cook, 1974), it’s a widely used explosive and accounts for 80% of all blasts that occur in the United States (Green, 2006). The reason that ANFO is so widely used in industries such as mining, quarrying, and demolition, is due to ANFO being low-cost and easy to use (Cook, 1974).
What are Some Issues with Ammonium Nitrate?
Issues with ammonium nitrate arise from storage and handling. In the event of a fire, ammonium nitrate can melt and releases toxic fumes and if it reacts with water, it can form ammonium hydroxide solution which can cause irritation and burns. Thus, it is evident that ammonium nitrate has deadly reciprocations if not handled and stored in a safe and secure condition (Health and Safety Executive, 2018). Furthermore, due to ammonium nitrate being an oxidising agent when it comes into contact with other substances it can react with it can cause large explosions. This has led to incidents such as the Oklahoma bombing which used an ammonium nitrate bomb to destroy a building and killed 168 people (CNN, 2018).
Due to the risk of ammonium nitrate being used as a bomb for acts of terror, the sale of ammonium nitrate is closely monitored; for example, in the United States where the department of homeland security has multiple statutory authorities working for it that regulate the production and sale of ammonium nitrate for security purposes (Dana A. Shea, 2013). Due to the monitoring of ammonium nitrate, it is becoming increasingly harder to buy ammonium nitrate-based fertilisers on a small scale, this has affected the ammonium nitrate market as alternatives such as urea can be used in its place. However, using urea as a fertiliser is problematic as it can lose 40% of its nitrogen content if there is no rainfall within 2 days of it being used.
The Future of Ammonium Nitrate
In the future the restrictions that governments have on ammonium nitrate-based fertilisers may tighten; innovation will be the key for the future of fertilisers that rely on ammonium nitrate. A new technology that has recently been produced is a fusion of ammonium nitrate and ammonium sulfate, this fusion produces a product that is less explosive and is more efficient as a fertiliser. It is not regulated so it can be bought by anyone (FUSN, 2018). However, the drawback with this fusion is that it is relatively new and hasn’t been on the market long enough to become an established competitor for ammonium nitrate, which has been used in fertilisers for a very long time and will continue to be used around the world for the foreseeable future.
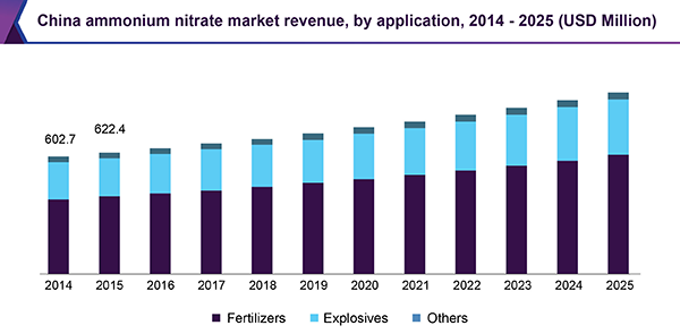
Even with competition and innovations, the market for ammonium nitrate will continue to grow, this is due to continued high demand for explosives and fertiliser globally and the simplicity of producing ammonium nitrate and using it. The increased demand for explosives is due to increased military activity around the world and the increased mining in countries such as the USA, India, and Argentina for valuable metals and minerals. This has resulted in more mines opening which require blasting products, thus increasing the demand for ammonium nitrate. The high demand for fertilisers by the agricultural industry is due to the increasing global population, especially in China which uses the largest amount of nitrogen-based fertiliser in the world due to its high population which is increasing (figure 4).
References
CNN. (2018, March 25). Oklahoma City Bombing Fast Facts. Retrieved from CNN: https://edition.cnn.com/2013/09/18/us/oklahoma-city-bombing-fast-facts/index.html
Cook, M. A. (1974). The Science of Industrial Explosives. Salt Lake City: ireco chemicals.
Crop Nutrition. (2018). Nitrogen in Plants. Retrieved from Crop Nutritionhttps: https://www.cropnutrition.com/efu-nitrogen
Dana A. Shea, L.-J. S. (2013). Regulation of Fertilizers: Ammonium Nitrate and Anhydrous Ammonia.Washington: Congressional Research Service.
Encyclopedia. (2018, November 4). Ammonium Nitrate. Retrieved from Encyclopedia.com: https://www.encyclopedia.com/science/academic-and-educational-journals/ammonium-nitrate
FUSN. (2018). Product Data shee 26-0-0-14s. Retrieved from FUSN: http://simplotfusn.com/documents/FUSN-PDS.pdf
Global Information. (2018). Global Ammonium Nitrate Market 2018-2022. Retrieved from Global Information: https://www.giiresearch.com/report/infi666003-global-ammonium-nitrate-market.html
Grand View Research. (2017). Ammonium Nitrate Market Analysis By Application (Fertilizers, Explosives), By Region (North America, Europe, Asia Pacific, CSA, MEA), Competitive Landscape, And Segment Forecasts, 2018 – 2025. Retrieved from Grand View Research: https://www.grandviewresearch.com/industry-analysis/ammonium-nitrate-market
Green, E. M. (2006, june). Explosives regulation in the USA. Retrieved from Crowell: https://www.crowell.com/documents/DOCASSOCFKTYPE_ARTICLES_408.pdf
Health and Safety Executive. (2018). STORING AND HANDLING AMMONIUM NITRATE. Retrieved from Health and Safety Executive: http://www.hse.gov.uk/pubns/indg230.pdf
madehow. (2018). Fertilizer. Retrieved from How Products Are Made: http://www.madehow.com/Volume-3/Fertilizer.html
Pubchem. (2018). Ammonium Nitrate. Retrieved from Pubchem: https://pubchem.ncbi.nlm.nih.gov/compound/ammonium_nitrate#section=Top
Study. (2018). Ammonium Nitrate: Uses & Formula. Retrieved from study.com: https://study.com/academy/lesson/ammonium-nitrate-uses-formula.html
The conversation. (2020). Ammonium nitrate and iodine: a look back at the explosive history of two essential substances. Retrieved from The conversation: https://theconversation.com/ammonium-nitrate-and-iodine-a-look-back-at-the-explosive-history-of-two-essential-substances-146448
United States Environmental Protection Agency. (2018). Ammonium Nitrate. Retrieved from https://www3.epa.gov/ttnchie1/ap42/ch08/final/c08s03.pdf?fbclid=IwAR2ZJNM6V7HjFAQBOoHm6TxQmXDdYT2hzczUF0YEXOQ1uTbT6S7bLRW9xQ8

Dr. Adam Zaidi, PhD, is a researcher at The University of Manchester (UK). His doctoral research focuses on reducing carbon dioxide emissions in hydrogen production processes. Adam’s expertise includes process scale-up and material development.’


