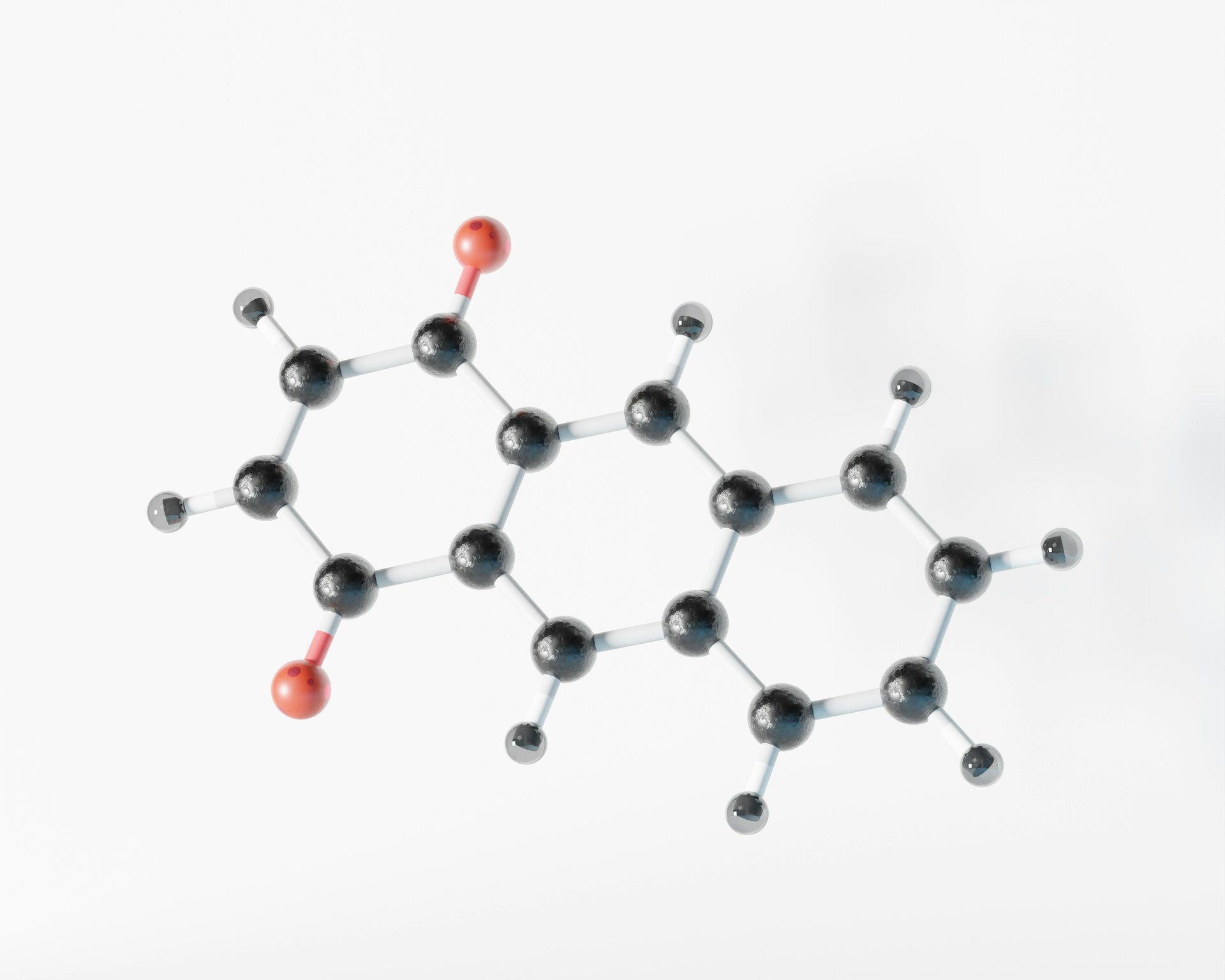
Chemists at the University of Oxford have successfully created and characterized a cyclo[48]carbon ring that remains stable in liquid solution at room temperature. This achievement, published in Science, represents a rare advance in the field of carbon allotropes, since most previously known forms could only exist in extreme conditions.
The research was led by Dr. Yueze Gao as first author with senior supervision by Professor Harry L. Anderson, both at Oxford’s Department of Chemistry. The team included collaborators from the University of Manchester, University of Bristol, and the Central Laser Facility at Rutherford Appleton Laboratory. Amongst the co-authors are Prakhar Gupta, Igor Rončević, Coral Mycroft, Paul J. Gates, and Anthony W. Parker.
Au, S., Gauthier, J. R., Kumral, B., Filleter, T., Mabury, S., & Golovin, K. (2025). Nanoscale fletching of liquid-like polydimethylsiloxane with single perfluorocarbons enables sustainable oil-repellency. Nature Communications, 16(1), 6789. https://doi.org/10.1038/s41467-025-62119-9
Cyclo[48]carbon is a hollow molecular ring of 48 carbon atoms bonded in an alternating pattern of single and triple bonds. Traditionally, such carbon rings are highly reactive and unstable in ordinary environments. The innovation here is that the carbon ring has been threaded through three other large molecular loops, called macrocycles, forming a structure known as a “[4]catenane.” These macrocycles protect the carbon ring, reducing its susceptibility to breaking apart or reacting undesirably.
Dr. Yueze Gao (Department of Chemistry, University of Oxford) stated,
“Achieving stable cyclocarbons in a vial at ambient conditions is a fundamental step. This will make it easier to study their reactivity and properties under normal laboratory conditions.”
One of the more striking results is that the cyclo[48]carbon [4]catenane remains stable in solution at ~20 °C with a half-life of about 92 hours. That long a half-life in liquid at ambient temperature is almost unheard of for cyclocarbons, which until now have been observable only in gas phase, on surfaces, or at cryogenic temperatures (~4-10 K).
To confirm that the molecule is what the researchers claim, they used a combination of characterization techniques. Mass spectrometry confirmed the expected molecular weight, UV-visible spectroscopy and Raman spectroscopy provided insight into electronic structure and vibrational modes, and notably, ^13C nuclear magnetic resonance (NMR) gave a single strong resonance for all 48 sp-hybridized carbon atoms. That indicates that each carbon atom is in an equivalent chemical environment—strong evidence that the ring has formed properly and is symmetric in those conditions.
Although the result is promising, there remain open questions. The protective macrocycles help stability, but further study is needed to understand how the molecule behaves under mechanical stress, in different solvents, and over extended durations. Also, its reactivity—how it might degrade or participate in chemical reactions—needs more examination. Such data will be essential before any potential applications are considered.
From an applications standpoint, this advance opens doors for deeper study of carbon-based molecular rings under “normal” lab conditions. Possible areas of interest include molecular electronics, where conductive or semiconductive carbon structures at the nanoscale may have uses; fundamental studies of bonding and stability in conjugated carbon systems; and perhaps future materials whose properties derive from exotic carbon architectures. But as with many breakthroughs, the transition from discovery to applied technology will require work. Ensuring reproducibility, scaling synthesis, understanding durability, and assessing cost are all likely to pose challenges.
The stabilization of cyclo[48]carbon as a [4]catenane at room temperature represents a milestone in carbon chemistry. By threading the ring through macrocycles, the researchers have made a previously fragile structure accessible for study under ordinary lab conditions. As researchers build on this work, we may see new types of carbon-based molecular devices or materials that were once purely theoretical.

Adrian graduated with a Masters Degree (1st Class Honours) in Chemical Engineering from Chester University along with Harris. His master’s research aimed to develop a standardadised clean water oxygenation transfer procedure to test bubble diffusers that are currently used in the wastewater industry commercial market. He has also undergone placments in both US and China primarely focused within the R&D department and is an associate member of the Institute of Chemical Engineers (IChemE).