What Is Water Softening And What Are Its Advantages
Water softening is used to remove the hardness from water, which is primarily caused by the presence of minerals like calcium and magnesium. The use of water softening has several benefits:
- Prevention of Scale Buildup: Hard water can lead to the accumulation of scale inside pipes, boilers, and appliances like washing machines and water heaters, which can reduce their efficiency and lifespan.
- Improved Cleaning: Soft water enhances the effectiveness of soap and detergents, resulting in cleaner dishes, laundry, and surfaces, and can reduce the amount of soap needed for tasks.
- Appliance Longevity: Appliances that use water, like dishwashers and coffee makers, last longer and work more efficiently when scale buildup is minimized by using softened water.
- Better Water Quality: Softened water can improve the taste and smell of drinking water in some cases, especially if the hardness was causing a noticeable taste or odor.
- Skin and Hair Health: Hard water can leave skin feeling dry and hair looking dull due to excess minerals. Soft water can alleviate these issues, leading to softer skin and more manageable hair.
Water softening is thus an important process for residential, commercial, and industrial settings to protect infrastructure and improve the efficiency and effectiveness of cleaning and heating processes.
What Is Ion Exchange?
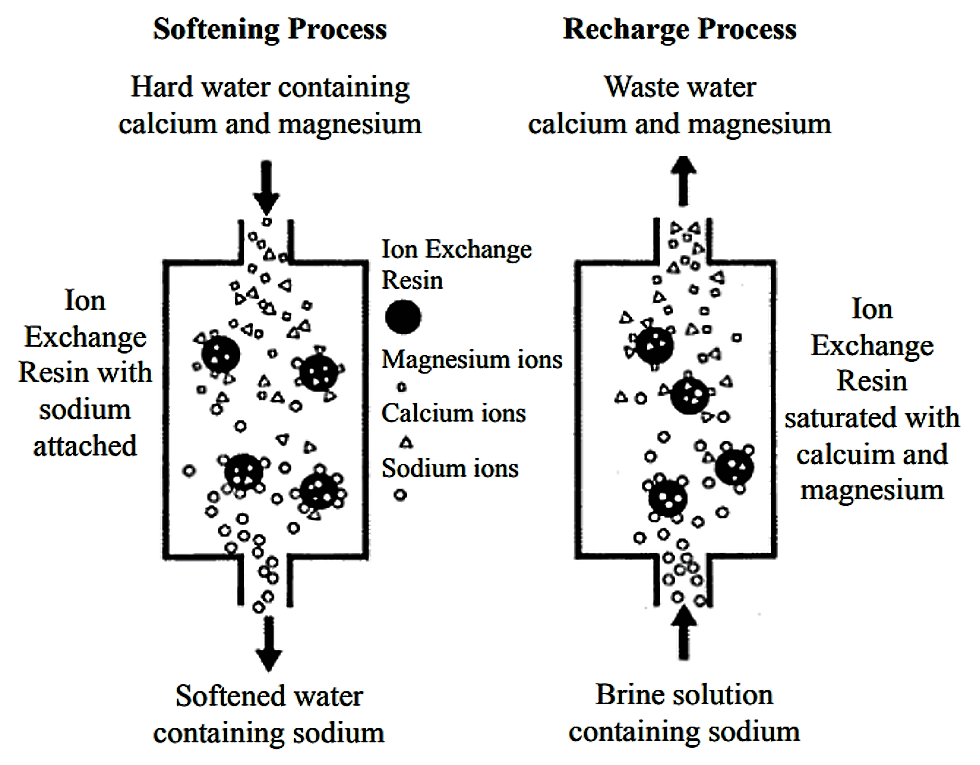
Ion exchange serves a broad spectrum of applications, yet it is chiefly employed for water softening by employing gel resins. This process is done in a cyclical sequence within a fixed bed, made up of four primary phases, Loading phase, Displacement Phase, Regeneration phase and finally Washing Phase.
Ion Exchange Phases In Water Softening
Loading Phase
In this initial step, the solution flowing through the resin bed undergoes ion exchange. The resin selectively absorbs divalent cations, specifically calcium (Ca2+) and magnesium (Mg2+), from the water. As a result of this exchange, an equivalent amount of sodium ions (Na+) is released from the resin into the water. The efficiency of this phase hinges on the rate at which equilibrium between the resin and the solution is achieved. The desired outcome is the effective replacement of hardness-causing ions with sodium ions from the resin, thereby softening the water.
Displacement Phase
Following the loading phase, the next step involves preparing the resin for regeneration. This is done by displacing the ions that have accumulated during the loading phase with a concentrated sodium chloride solution, facilitating the removal of divalent cations from the resin sites. The goal is to prepare the saturated resin for regeneration by displacing the hardness ions with a high concentration brine solution.
Regeneration Phase
During this critical step, the previously absorbed divalent ions are replaced by sodium ions from the regenerating solution. This replenishes the resin’s ion exchange capacity, making it ready for another cycle of ion exchange. The aim here is to restore the ion exchange capacity of the resin by replacing the hardness ions with sodium ions, using a concentrated salt solution.
Washing Phase
The final stage of the cycle entails thoroughly washing the resin to eliminate any residual regenerating solution. This ensures that the resin is clear of high-concentration sodium ions, which could otherwise contaminate the softened water in the subsequent loading phase. The final objective is to remove any excess brine from the resin, ensuring it is ready for the next cycle without introducing excessive sodium into the treated water.
This ion exchange cycle is optimally modeled when the mass transfer between the solution and the resin is rapid, allowing for the system to reach equilibrium quickly, a condition often described by a specific equilibrium expression. This expression accounts for the selectivity of the resin towards divalent over monovalent ions, ensuring a favorable exchange process.
The progression of the ion exchange is well approximated using simple stoichiometric or shock-wave front theory for adsorption, assuming plug flow. This means that the front of the ion exchange wave moves down through the bed in a uniform front, with the resin behind or upstream of the front remaining in equilibrium with the feed composition. The end of the cycle is marked by a breakthrough when the ion exchange front reaches the end of the bed, indicating that the bed’s capacity for ion exchange has been fully utilized and necessitates regeneration.

Adrian graduated with a Masters Degree (1st Class Honours) in Chemical Engineering from Chester University along with Harris. His master’s research aimed to develop a standardadised clean water oxygenation transfer procedure to test bubble diffusers that are currently used in the wastewater industry commercial market. He has also undergone placments in both US and China primarely focused within the R&D department and is an associate member of the Institute of Chemical Engineers (IChemE).


