What Is An Ideal Gas?
An ideal gas (or perfect gas) conforms to the idealised relationship between temperature, volume and pressure called the ideal gas law. The ideal gas law contains both Boyles’ law and Charles’ law as special cases and states that for a specified quantity of gas of volume, V and pressure, P it is proportional to the absolute temperature (equation 1), with k being a constant. This is called the equation of and is enough to describe the gross behaviour.
PV = kT
Equation 1: Ideal gas state equation for a specified quantity of gas.
(1.1)
For any gas, another form of state equation can be used, according to Avogadro’s number, if the constant specifying the quantity of gas is expressed in terms of the number of molecules of gas. This is done by using the mass unit the gram mole (molecular weight expressed in grams), and the equation of state of n gram-moles of a perfect gas changes (equation 2), with R being the universal gas constant, with a value of 8.314 joules per gram-mole-kelvin (Gregersen, 2020).
PV = nRT
Equation 2: Equation of state of an ideal gas for any gas.
(1.2)
Kinetic Theory Assumptions About Ideal Gases
An ideal gas or a perfect gas and obeys the ideal gas law (equation 2) it is a good describer for the behaviour of the many gases under many condition types, although there no such thing as an ideal gas in the real world, real gases will be discussed in greater detail in another post.
The ideal gas law can be derived from the kinetic theory of gases and relies on the key assumptions:
- No (or entirely negligible) intermolecular forces between the gas molecules/atoms,
- Molecules/atoms are points and do not occupy any volume.
Other Assumptions are:
- Molecules behave as rigged spheres,
- Molecules/atoms move randomly in straight lines,
- Pressure is caused by collisions between molecules/atoms and the walls of the container,
- Temperature is proportional to the average kinetic energy of the molecules/atoms,
- Molecules/atoms are equally sized.
Summary of Gas Laws:
- Boyles’ law – Isothermal expansion/compression
An ideal gas obeys Boyles’ law at all pressure and for a fixed amount of gas, the pressure and volume at constant temperature are related by:
PV = constant or P1V 1 = P2V 2
- Charles’ law – Isobaric compression/expansion
Charles’ law identifies absolute zero and for a fixed amount of gas, the volume and temperature of a fixed amount of gas at constant pressure are related by, with k being the constant (figure 1):
V = k x T
This law shows that increasing the temperature will increase the kinetic energy of the gas molecules, resulting in the gas occupying a greater volume at the same pressure.
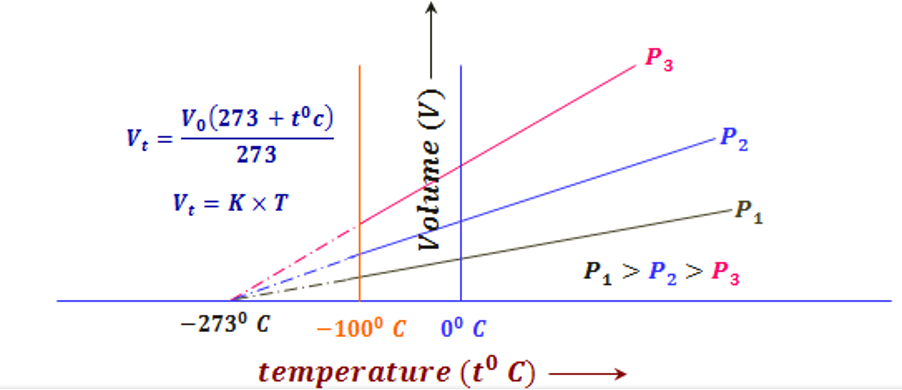
Figure 1: Volume Temperature Graph for Gases (Priyamstudycentre, 2020).
- Gay-Lussac law – for a fixed amount of gas, the pressure and volume are directly proportional to the gas’s absolute temperature, with k being a constant.
When the temperature is in kelvin:
P = k x Tk
When the temperature is in degrees Celsius:
P = k x (Tk + 273.15)
- Avogadro’s’ principle – Equal volumes of gases at the same temperature and pressure contain the same number of molecules, with k being a constant and n is the number of moles
V = k x n
Mixtures of Gases (Daltons Law)
When there is a mixture of gases, the partial pressure, Pi, of a gas is the mole fraction, xi, multiplied by the total pressure (equation 3).
Pi = xiP
Equation 3: Partial pressure equation.
(1.3)
The mole fraction of a gas within the mixture is a fraction of the total number of moles in the mixture (equation 4).
xi = ni/n
Equation 4: mole fraction equation.
(1.4)
Daltons’ law can be used to find out the total pressure of a system or one partial pressure if the total pressure and the rest of the partial pressures are given. This is because Daltons’ law states that the total pressure of a perfect gas is the sum of the partial pressures of the gases (equation 5).
P = Pi + Pj + ….
Equation 5: Daltons’ law.
(1.5)
References
Gregersen, E. (2020). Gas laws. Retrieved from Encyclopedia Britannica: https://www.britannica.com/science/gas-laws
Priyamstudycentre. (2020). Gases. Retrieved from Priyamstudycentre: https://www.priyamstudycentre.com/2019/02/physical-properties-gases.html

Dr. Adam Zaidi, PhD, is a researcher at The University of Manchester (UK). His doctoral research focuses on reducing carbon dioxide emissions in hydrogen production processes. Adam’s expertise includes process scale-up and material development.’