What Is A Material Balance?
Material balances also called mass balances, is an application of the law of conversation of mass, which states matters is neither created nor destroyed. Material balances are vital for chemical engineers as they are the basis of process design and allow the design of units themselves as they determine the quantities of raw materials required for the quantity of product required.
The material balance can be seen as the accounting for materials that enter and leave a process and accounts for all materials even if they undergo chemical reactions, separation, heating, cooling, mixing, drying or any other operation (excluding nuclear reactions) that occurs in the system.
Material balances are a really useful tool for studying plant; operations, troubleshooting, checking actual plant performance versus the design performance (what the plant works at against what it was designed for), can extend the amount of data from plant instrumentation, checks if instruments are calibrated correctly and can help locate sources of material loss.
Key Definitions
A list of definitions that must be understood when doing material balances:
- Steady-state – Conditions at all points within the process are constant with time.
- Unsteady state – Conditions within the process vary with time.
- System boundary – the imaginary box is drawn around the system that is being analysed.
- Open system (or flow system) – Materials crosses the system boundary during the process.
- Closed system – no materials cross the system boundary except the beginning and end of the process, and changes take place within the system and heat may be transferred.
System Boundary
The system or region needs to be defined by an imaginary closed box which is called the system boundary, which should always be drawn to avoid making any mistakes (figure 1). A system can be one single process unit, a collection of process units or an entire process.
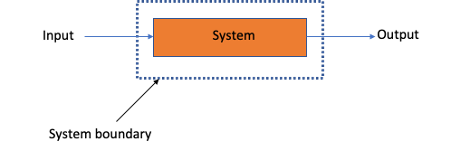
Figure 1: How to define a system boundary on a system
Material Balance Equation
As stated previously the material balances obey the law of conversation of mass, thus mass in is equal to mass out. The equation that needs to be remembered by every chemical engineer at all levels is:
Accumulation = (Mass in – Mass out) + Generation – Consumption
- Accumulation – The change in quantities of materials inside the system
- Mass in – Materials entering the system that cross the system boundary
- Mass out – Materials leaving the system that cross the system boundary
- Generation – Materials produced by a chemical reaction that takes place within the system
- Consumption – Materials used by a chemical reaction that takes place within the system
Simplification For The Material Balance Equation
In certain cases, the material balance equation will be affected:
- No net accumulation – as the process is in steady-state or when you have a batch vessel which starts empty and then ends empty, so no accumulation within the process, the equation becomes:
In + Generation = Out + Consumption
- No reaction – No materials are used up such as when blending, washing or drying. Thus, no generation of a new product of consumption of starting materials, the equation becomes:
Accumulation = In – Out
- No net accumulation or reaction – nothing is consumed, and no new products are created, and no materials remain in the process, the equation becomes:
In = Out
Choosing A Basis
A basis is a reference point that you can choose when wanting to solve a problem where the information gave is limited or purposely missed out such as an exam type question. A good choice of basis makes material balances much easier to solve, and can be a flow rate, a unit of time or an amount.
When choosing your basis, always state your basis clearly to remove any issues that could arise from colleagues not understanding where your values came from, and for exam markers to be able to get the marks given for choosing a basis.
Other things that need to be considered are; the units to be used, the most convenient basis value to be used and what the question is asking. Having this checklist will make creating a basis straightforward and minimise mistakes.
In a scenario where one stream has enough data, then use that stream as it has the most data and if no data then assume a basis for a stream with known components, and when mass fractions are known then choose either the total mass or the mass flow rate as the basis. Similarly, if mole fractions are given then choose the total number of moles or the molar flow rate.
When having to choose a basis for a continuous steady-state process a sensible choice for the basis is based on the amount of material entering or leaving the system within a period i.e. Kg/hr. For a batch or semi-batch process then base the basis on the inlet amount at the feed initially or the output at the end of the process i.e. Kg/batch.
Procedure For Solving A Material Balance
- Carefully analyse the situation now what you are being asked to find or work out.
- Label all quantities on the block flow diagram (BFD) or process flow diagram (PFD) using the units given and doing any conversions to make sure all units are the same.
- Choose your basis, keep this simple by using easy numbers i.e. 100 kg or 1 hour.
- Draw your system boundary.
- State any assumptions made.
- Draw a calculations table to make it easier to see the values you have worked out
- Check your solution to make sure it makes sense!
Material Balance Example
A centrifuge is used to separate particles of a diameter of 0.5 – 50 mm from a liquid. Yeast cells are recovered from a broth (a liquid mixture containing cells) using the centrifuge. Find out the amount of cell-free discharge if per hour if 1000 L-1 is the feed stream that contains 500 mg cells L-1 with a product stream that contains 50 wt% cells. Assume a density of 1 g cm 1.
Try to do this question without looking at the answer, if you can do this first time without looking at the answer it will be very impressive, but if you cannot don’t worry getting used to material balance questions takes time.
Hint: Use the procedure to solve this question and only use the information you need to be careful of red herrings!

Dr. Adam Zaidi, PhD, is a researcher at The University of Manchester (UK). His doctoral research focuses on reducing carbon dioxide emissions in hydrogen production processes. Adam’s expertise includes process scale-up and material development.’




